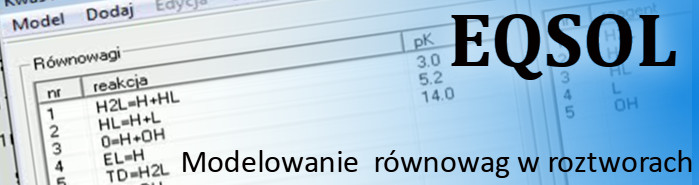
Functioning of the program is based on the model of symbolic equations depicting particular equilibria.
Owing to the adjustment of the calculated titrant curves to the experimental curves, by the use of nonlinear method of the least squares, the program generates theoretical titration curve and estimates all quantities describing the model, including the type of solution: ideal, Debye-Hueckel or Davies.
Additional tools allow further specific analysis of acquired data. Simulation Function can be particularly useful in didactics to generate titrants’ curves of any systems.
Supplementary informational materials: